Results
EC and pH in Urmia Lake and Van Lake waters with and without dilutio
In Table 1 and Table 2, the average values of pH and EC of ULW and VLW waters with and without centrifuging the samples are presented. The comparison of two tables shows that removing suspended particles from water samples didn't have significant effect on pH and EC values of two waters. Furthermore, to see the effects of dilution with distilled water on pH and EC of the water samples, 10 and 100 times dilution coefficients were applied. According to Table 1, diluting ULW water increased the pH of the sample but didn't have significant effect on the VLW water sample. As the pH of the distilled water was neutral, diluting ULW with initial pH of 7.04, resulted in decrease of H activity and increase of pH. In case of VLW, due to existence of CO3- and CO32- ions and their buffering capacity, dilution didn't have significant effect on pH. EC values, as expected, decreased in both water samples by diluting them, but not exactly in a 1:1 linear relation due to high EC values of both water samples.
Table 1. The average values of the pH and EC of water samples (without centrifuging)
b. EC and pH results from mixing Urmia and Van Lakes
EC and pH results from mixing ULW and VLW water samples with different ratios are presented in Table 3. According to Table 3, mixing ULW with VLW water will increase water pH in any ratios of two waters.
Considering EC changes with adding different amounts of VLW to ULW, it worth saying that according to Table 3, water EC won't change much even if VLW water content rise to two times higher than ULW. Adding VLW water in low volumes will probably raise the EC of the resulting water due to ionic components and salt clusters separations in Urmia supersaturated Water as a result of dilution.
c. EC and pH results from mixing Urmia and Van Lakes and in equilibrium with Urmia Lake salt crusts
Results from mixing to waters with different ratios in equilibrium with ULW salt crust showed that even in case of adding VLW in high volumes to ULW, in the presence of salt crust, EC will raise from 158 (only ULW water with USC) to 212 (60:140 U:V). This means that under natural condition of ULW, adding VLW to ULW will results in increasing water pH, dissolving more salts and increasing EC of the new water.
d. Basic chemical analysis of water mixing with and without Urmia salt crust
Results from chemical analysis of water mixtures for basic anions and cations are presented in table 5.
Na and K
According to Table 5, Na and K concentrations in ULW are much higher than VLW. In different ratios of two waters, by increasing the content of ÚLW, Na and K concentrations were also increased. The highest amount of Na were obtained in 140:60 U:V in equilibrium with USC. This shows that USC also has great amounts of Na in its composition. Similar thing wasn't true in case of K, which means that probably USC doesn't contain components with K in their structure. The highest amount of K was observed in initial ULW and in all other water mixtures, with or without USC, K conc. Decreased.
e. XRD analysis
Urmia Lake and Van Lak
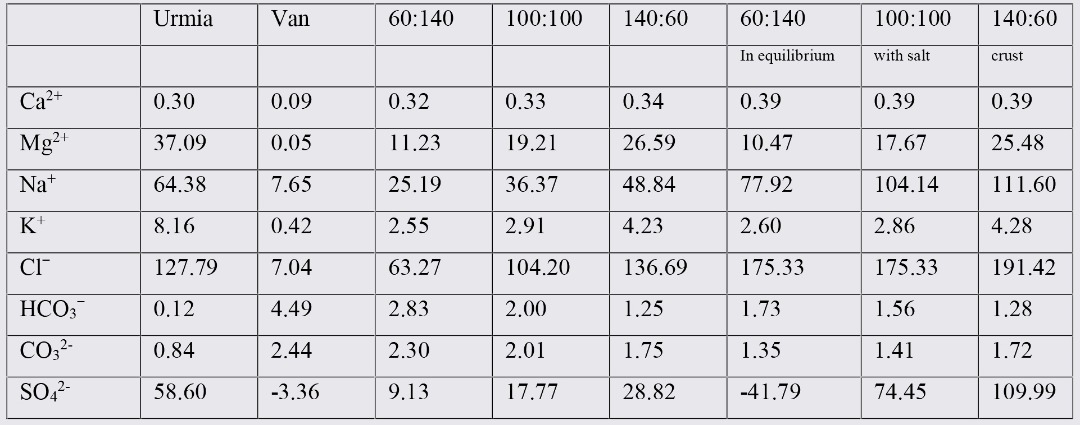
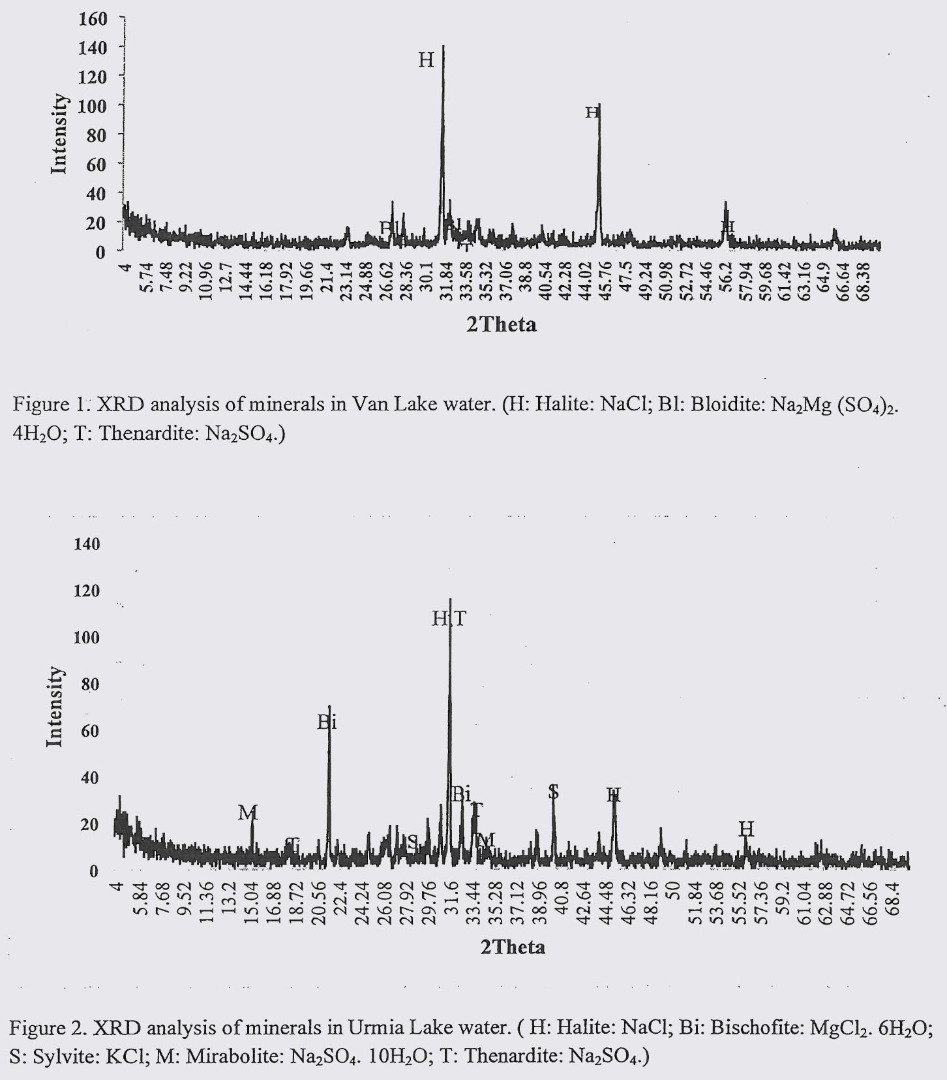
Results from XRD analysis of VLW and ULW are presented in figures 1 and figure 2. In VLW common minerals were Halite (NaCl); Bloidite (Na2Mg (SO4)2: 4H2O); Thenardite (Na2SO4). In ULW the prevailing minerals were detected to be Halite (NaCl); Bischofite (MgCl2. 6H2O); Sylvite (KCl); Mirabolite (Na2SO4.10H2O) and Thenardite (Na2SO4). Comparing the minerals available in these two waters, it could be said that in both waters similar anions (Cl and SO4) are available in minerals structures. Among cations, Na and Mg were available in both waters but K was only observed in ULW minerals.
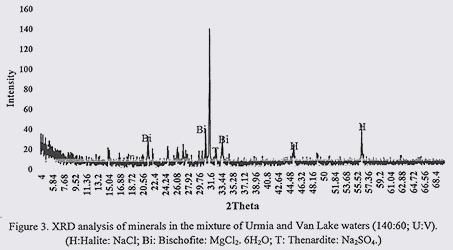
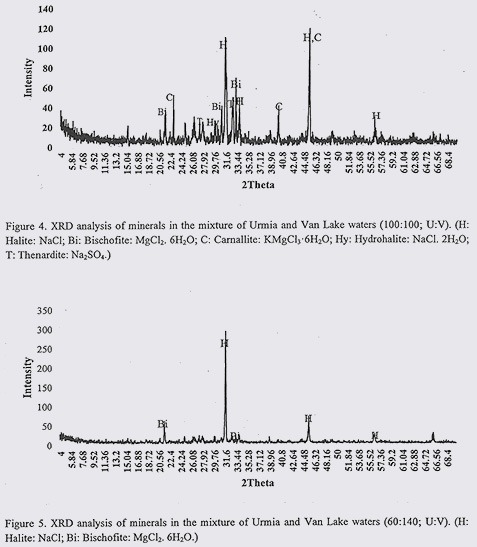
Results from XRD analysis of VL and UL water mixtures with different mixing rations are presented in figures 3 to 5. According to these findings, in water sample of 140:60 (U:V), common minerals were Halite (NaCl); Bischofite (MgCl2. 6H2O) and Thenardite (Na2SO4). In 100:100 (U:V) water sample, common minerals were appeared to be Halite (NaCl); Bischofite (MgCl2. 6H2O); Carnallite (KMgCl3.6H2O); Hydrohalite (NaCl. 2H2O) and Thenardite (Na2SO4). In 60:140 (U:V) prevailing minerals were as follow: Halite (NaCl) and Bischofite (MgCl2. 6H2O). Considering these results, in all of three water mixtures, NaCl and MgCl2 were among common minerals. By increasing the content of ULW in water samples, Na2SO4 also formed which were absent from 60:140 (U:V) water sample. In equal contents of both waters, Carnallite (KMgCl3.6H2O) were also observed.
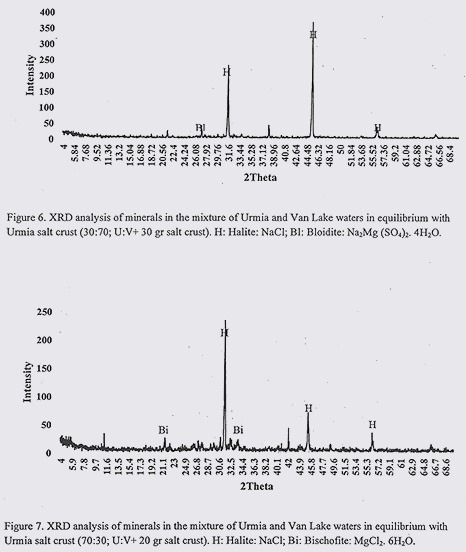
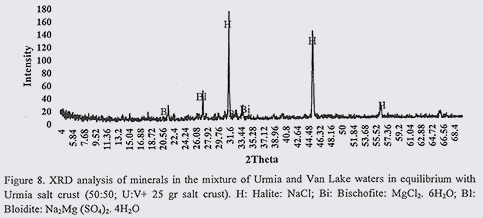
Results from XRD analysis of VL and UL water mixtures in equilibrium with salt are presented in figures 5 to 7. Before talking about common minerals in water samples and their similarities and dissimilarities, it should be mentioned that by increasing the content of the VLW in the mixed water samples, the solubility of USC increased (as expected due to low salinity of VLW), therefore necessary salt content to establish equilibrium, increased by increasing the content of the VLW.
According to XRD results, in water sample of 30:70 (U:V in equilibrium with 30gr USC), common minerals were Halite (NaCl) and Bloidite (Na2Mg (SO4)2. 4H2O). In 50:50 (U:V in equilibrium with 25 gr) water sample, common minerals were appeared to be Halite (NaCl) and Bischofite (MgCl2. 6H2O). In 70:30 (U:V. in equilibrium with 20gr USC) prevailing minerals were as follow: Halite (NaCl) and Bischofite (MgCl2. 6H2O). Considering these results, in all of three water mixtures, the same as previous section, NaCl was common mineral. In sample with highest content of VLW (hence with higher content of salt), Na2MgSO4, replaced MgCl2.6H2O (which were detected in other two samples).
According to the above context, in natural condition, in case VLW be transferred to UL, great amount of salt deposited in the bed of the UL will be dissolved, so planners should consider that water transferring won't reduce the salinity of the UL nor bring back the ecosystem existed before drying of the UL. But, they should think of the problems that area can face because of the lack of water cover in barren saline lands all around the UL.
✓ Will addition of Van Lake water improve the chemical condition of Urmia Lake?
o Not necessarily, but considering the current situation of Urmia Lake, water with better or worse chemical condition is pointless.
✓ Will water transfer from Van Lake to Urmia Lake revive Artemia?
o Due to huge amounts of salt in Urmia Lake bed, probably there is no hope in that.
✓ Will water transfer from Van Lake to Urmia Lake cause serious threat to the area?
o Doesn't seem so.
✓ Can water transfer from Van Lake help to improve the existing condition of the area?
o Although the question is not in the area of this research, but similar experiences from dried lakes like Owens Lake says that transferring water from Van L to Urmia L will at least cover the barren playa surfaces which are very susceptible to wind erosion and in time, will decrease the environmental hazards related to dust induced problems.
✓ Does water transfer from Van L to Urmia L be recommended?
o Answering to this question needs other studies in the several aspects of economic, political and social consequences of water transfer. However, in case the project is justifiable, the advantages of water transfer will probably be higher than its disadvantages.
✓ Which part of Urmia Lake has higher priority for water transfer from Van Lake?
o The northern section of Urmia Lake is closer and thus will be more economical to consider. It is possible to separate the northern section along the Tabriz-Urmia highway. This section will be more salty as compare to the southern part that will receive fresh water from rivers. In this case it is feasible to create some less salty water bodies in the southern section and increase the chance of biological revival.
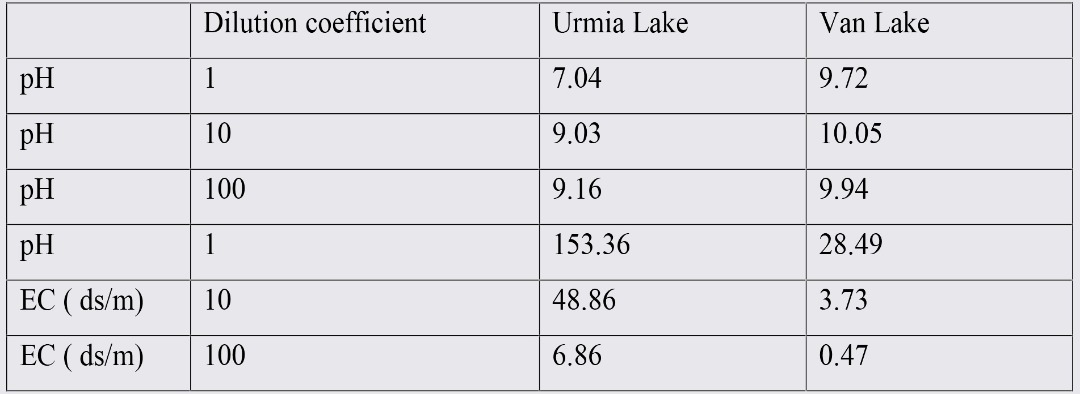
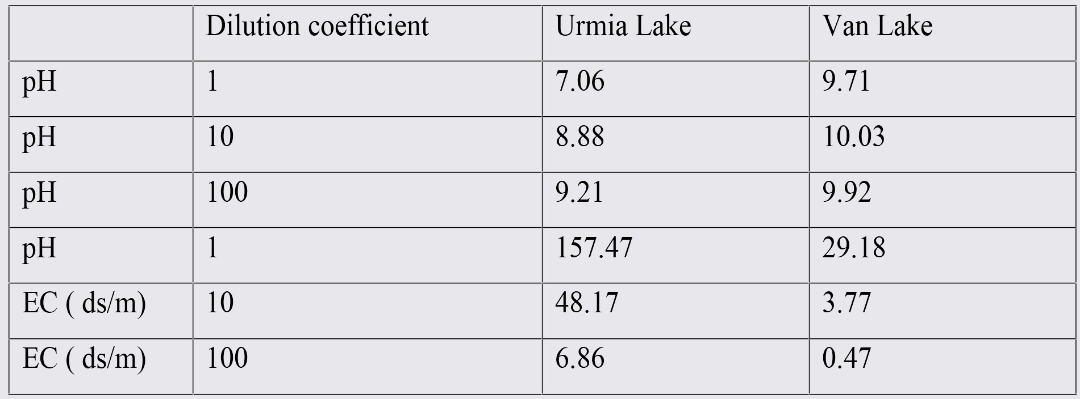
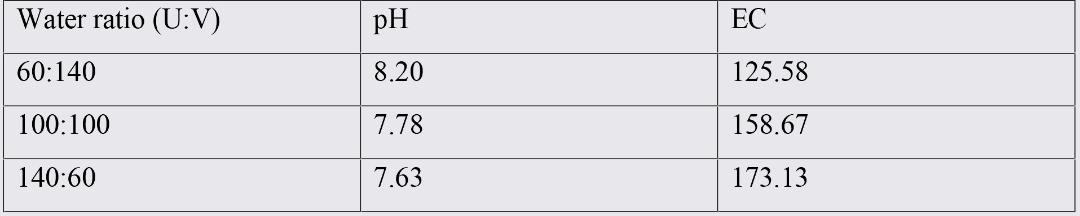
Table 4. The average values of the pH and EC in mixed water samples in equilibrium with salt crust

Table 5 - The average concentrations of cations and anions*
*the concentration of anions and cations are presented in g/L